

The adaptr package simulates adaptive (multi-arm,
multi-stage) clinical trials using adaptive stopping, adaptive arm
dropping and/or response-adaptive randomisation.
The package has been developed as part of the INCEPT (Intensive Care Platform Trial) project, which is primarily supported by a grant from Sygeforsikringen “danmark”.
The easiest way is to install from CRAN directly:
install.packages("adaptr")Alternatively, you can install the development version from GitHub - this requires the remotes-package installed. The development version may contain additional features not yet available in the CRAN version (including preliminary functions) and may not be stable or fully documented:
# install.packages("remotes")
remotes::install_github("INCEPTdk/adaptr@dev")The basic functionality of adaptr is illustrated
below.
First, load the library and setup a trial specification using the
general setup_trial() function, or one of the special case
functions, setup_trial_binom() (used in the example) or
setup_trial_norm().
library(adaptr)
#> Loading adaptr package (version 1.2.0).
#> See 'help("adaptr")' or 'vignette("Overview", "adaptr")' for help.
#> Further information available on https://inceptdk.github.io/adaptr/.
# Setup a trial using a binary, binomially distributed, undesirable outcome
binom_trial <- setup_trial_binom(
arms = c("Arm A", "Arm B", "Arm C"),
true_ys = c(0.25, 0.20, 0.30),
min_probs = rep(0.15, 3), # Minimum allocation of 15% in all arms
data_looks = seq(from = 300, to = 2000, by = 100),
# Stop for equivalence at > 90% probability of differences < 5 %-points
equivalence_prob = 0.9,
equivalence_diff = 0.05,
soften_power = 0.5 # Soften allocation ratios
)
# Print trial specification
print(binom_trial, prob_digits = 3)
#> Trial specification: generic binomially distributed outcome trial
#> * Undesirable outcome
#> * No common control arm
#> * Best arm: Arm B
#>
#> Arms, true outcomes, starting allocation probabilities
#> and allocation probability limits:
#> arms true_ys start_probs fixed_probs min_probs max_probs
#> Arm A 0.25 0.333 NA 0.15 NA
#> Arm B 0.20 0.333 NA 0.15 NA
#> Arm C 0.30 0.333 NA 0.15 NA
#>
#> Maximum sample size: 2000
#> Maximum number of data looks: 18
#> Planned data looks after: 300, 400, 500, 600, 700, 800, 900, 1000, 1100, 1200, 1300, 1400, 1500, 1600, 1700, 1800, 1900, 2000 patients have reached follow-up
#> Number of patients randomised at each look: 300, 400, 500, 600, 700, 800, 900, 1000, 1100, 1200, 1300, 1400, 1500, 1600, 1700, 1800, 1900, 2000
#>
#> Superiority threshold: 0.99 (all analyses)
#> Inferiority threshold: 0.01 (all analyses)
#> Equivalence threshold: 0.9 (all analyses) (no common control)
#> Absolute equivalence difference: 0.05
#> No futility threshold (not relevant - no common control)
#> Soften power for all analyses: 0.5Simulate a single trial using a reproducible random seed:
trial_res <- run_trial(binom_trial, seed = 12345)
print(trial_res, digits = 3)
#> Single simulation result: generic binomially distributed outcome trial
#> * Undesirable outcome
#> * No common control arm
#>
#> Final status: inconclusive, stopped at final allowed adaptive analysis
#> Final/maximum allowed sample sizes: 2000/2000 (100.0%)
#> Available outcome data at last adaptive analysis: 2000/2000 (100.0%)
#>
#> Trial results overview:
#> arms true_ys final_status status_look status_probs final_alloc
#> Arm A 0.25 active NA NA 0.194
#> Arm B 0.20 active NA NA 0.656
#> Arm C 0.30 inferior 2000 0.007 0.150
#>
#> Esimates from final analysis (all patients):
#> arms sum_ys_all ns_all raw_ests_all post_ests_all post_errs_all lo_cri_all
#> Arm A 180 742 0.243 0.243 0.0161 0.213
#> Arm B 178 841 0.212 0.212 0.0141 0.185
#> Arm C 113 417 0.271 0.272 0.0221 0.230
#> hi_cri_all
#> 0.274
#> 0.240
#> 0.316
#>
#> Estimates from last adaptive analysis including each arm:
#> arms sum_ys ns raw_ests post_ests post_errs lo_cri hi_cri
#> Arm A 180 742 0.243 0.243 0.0159 0.213 0.275
#> Arm B 178 841 0.212 0.212 0.0141 0.185 0.241
#> Arm C 113 417 0.271 0.271 0.0215 0.230 0.316
#>
#> Simulation details:
#> * Random seed: 12345
#> * Credible interval width: 95%
#> * Number of posterior draws: 5000
#> * Posterior estimation method: medians with MAD-SDsSimulate multiple trials using a reproducible random seed:
# Simulate multiple trials - only 10 simulations for speed in the example
trial_res_mult <- run_trials(binom_trial, n_rep = 10, base_seed = 67890)
# Extract results in a tidy data.frame (1 simulation per row)
# See function documentation for details, including on arm selection in trials
# not ending with a superior arm
extr_res <- extract_results(trial_res_mult)
head(extr_res)
#> sim final_n sum_ys ratio_ys final_status superior_arm selected_arm
#> 1 1 2000 415 0.2075000 max <NA> <NA>
#> 2 2 600 139 0.2316667 superiority Arm B Arm B
#> 3 3 1000 237 0.2370000 superiority Arm B Arm B
#> 4 4 900 209 0.2322222 equivalence <NA> <NA>
#> 5 5 2000 441 0.2205000 superiority Arm B Arm B
#> 6 6 1900 431 0.2268421 superiority Arm B Arm B
#> sq_err sq_err_te
#> 1 NA NA
#> 2 7.853843e-04 NA
#> 3 4.190319e-05 NA
#> 4 NA NA
#> 5 3.422824e-06 NA
#> 6 4.852161e-05 NA
# Summarise trial results
# See function documentation for details, including on arm selection in trials
# not ending with a superior arm
res_sum <- summary(trial_res_mult)
print(res_sum, digits = 1)
#> Multiple simulation results: generic binomially distributed outcome trial
#> * Undesirable outcome
#> * Number of simulations: 10
#> * Number of simulations summarised: 10 (all trials)
#> * No common control arm
#> * Selection strategy: no selection if no superior arm
#> * Treatment effect compared to: no comparison
#>
#> Performance metrics (using posterior estimates from last adaptive analysis):
#> * Sample sizes: mean 1470.0 (SD: 559.9) | median 1550.0 (IQR: 1025.0 to 2000.0)
#> * Total summarised outcomes: mean 323.3 (SD: 110.6) | median 340.0 (IQR: 242.0 to 421.8)
#> * Total summarised outcome rates: mean 0.224 (SD: 0.013) | median 0.229 (IQR: 0.214 to 0.233)
#> * Conclusive: 70.0%
#> * Superiority: 50.0%
#> * Equivalence: 20.0%
#> * Futility: 0.0% [not assessed]
#> * Inconclusive at max sample size: 30.0%
#> * Selection probabilities: Arm A: 0.0% | Arm B: 50.0% | Arm C: 0.0% | None: 50.0%
#> * RMSE: 0.01330
#> * RMSE treatment effect: not estimated
#> * Ideal design percentage: 100.0%
#>
#> Simulation details:
#> * Simulation time: 0.471 secs
#> * Base random seed: 67890
#> * Credible interval width: 95%
#> * Number of posterior draws: 5000
#> * Estimation method: posterior medians with MAD-SDsPerformance metrics may also be calculated and returned in a tidy
data.frame (with bootstrapped uncertainty measures, if
requested) by the check_performance() function, and the
plot_convergence() function may be used to visually assess
stability of performance metrics according to the number of
simulations.
Plot trial statuses or history of trial metrics over time:
# Simulate multiple trials - 25 simulations only for speed
# sparse = FALSE is required for plot_history (but not plot_status)
trial_res_mult <- run_trials(binom_trial, n_rep = 25, base_seed = 67890, sparse = FALSE)
# Plot overall trial statuses according to the total number
# of patients randomised
plot_status(trial_res_mult, x_value = "total n")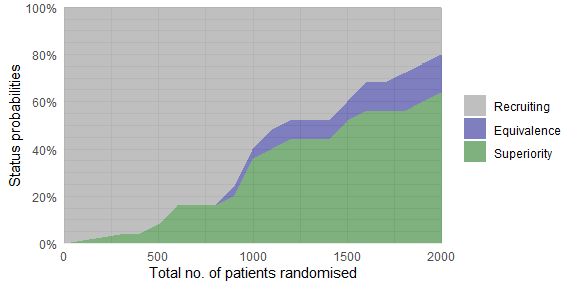
# Plot allocation probabilities at each adaptive look (requires sparse = FALSE)
plot_history(trial_res_mult)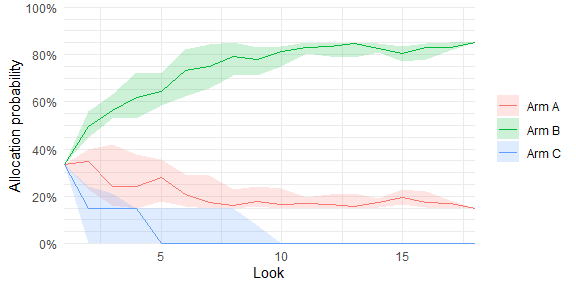
Plotting statuses for individual trial arms and other summary metrics is possible, too.
We use the GitHub issue tracker for all bug/issue reports and proposals for enhancements.
We welcome contributions directly to the code to improve performance as well as new functionality. For the latter, please first explain and motivate it in an issue.
Changes to the code base should follow these steps:
NEWS.md file to see the formatting)dev branch of adaptrIf using the package, please consider citing it:
citation(package = "adaptr")
#>
#> To cite adaptr in publications use:
#>
#> Granholm A, Jensen AKG, Lange T, Kaas-Hansen BS (2022). adaptr: an R
#> package for simulating and comparing adaptive clinical trials.
#> Journal of Open Source Software, 7(72), 4284. URL
#> https://doi.org/10.21105/joss.04284.
#>
#> A BibTeX entry for LaTeX users is
#>
#> @Article{,
#> title = {{adaptr}: an R package for simulating and comparing adaptive clinical trials},
#> author = {Anders Granholm and Aksel Karl Georg Jensen and Theis Lange and Benjamin Skov Kaas-Hansen},
#> journal = {Journal of Open Source Software},
#> year = {2022},
#> volume = {7},
#> number = {72},
#> pages = {4284},
#> url = {https://doi.org/10.21105/joss.04284},
#> doi = {10.21105/joss.04284},
#> }